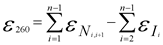For your convenience, please use our Oligo Analysis Tool or apply the formula below:
The extinction coefficient at 260 nm (e260) is a unique physical property of each oligonucleotide. It is defined as the absorbance at 260 nm of a 1 M aqueous solution measured at 20 °C in an optical cell with 1 cm pathway (Lambert-Beer's law).
Purinic bases show a higher absorption (OD260) than pyrimidinic bases. Interactions between neighbouring bases, as well as modifications that absorb at 260nm, also influence optical absorbance. As a consequence, the extinction coefficient strongly depends on oligonucleotide sequence and composition.
The most accurate method to calculate the extinction coefficient of an oligonucleotide is based on the nearest-neighbor model. The average error of calculated extinction coefficients was shown to be around 4 % using following formular: