The right answers to frequently asked questions
Find the answers to all our products and services by clicking the links below.
How do you synthesise your oligos?
We are using different synthesis machines with phosphoramidite chemistry (Caruthers, M.H. (1983) Tetrahedron Lett. 24:245-248).
The oligonucleotides are synthesised in 3' to 5' direction while attached covalently to a solid support (solid phase synthesis).The building blocks used for synthesis are DNA phosphoramidite nucleosides which are modified with different protection groups.
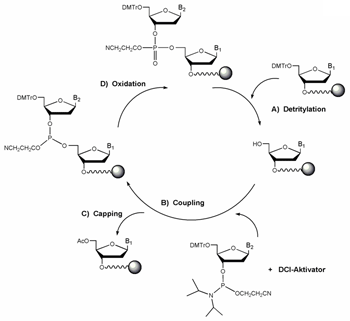
Synthesis starts with de-blocking (A) i.e. the cleavage of the 5' protection group dimethoxytrityl (DMT).
An activated phosphoramidite nucleoside couples with the free 5' hydroxyl function (B). Typically coupling efficiency is between 98% and 99.5%. Since the coupling efficiency is not 100%, a small percentage of truncated sequences is produced at every coupling step. If these failure sequences are allowed to further react, unwanted mutants would result.
This problem is overcome largely by capping the remaining free 5' hydroxyls through acetylation (C). Some molecules fail to cap and continue to participate in additional synthesis cycles, resulting in nearly full length molecules that contain internal deletions - the so-called (n-x) mer species.
After coupling, the DNA bases are connected by an unstable phosphite triester. It is converted to a stable phosphotriester linkage by oxidation (D). The oxidation step completes one cycle of oligo synthesis. DNA synthesis can continue with the removal of the DMT group at the 5' -end of the growing chain, starting another cycle of nucleotide addition.
Oligonucleotide cleavage from support and deprotection is achieved under alkaline conditions.
Does the sequence have an impact on synthesis success?
Eurofins Genomics has many years of experience in oligonucleotide synthesis. Occasionally we can identify oligonucleotides that are difficult to produce, due to their sequences. For example, oligonucleotides with a high percentage of "G" residues, especially stretches of "Gs", can be difficult to synthesise.
Moreover, oligos with four or more "Gs" in a row tend to aggregate and form a "guanine tetraplex" (Poon and MacGregor (1998) Biopolymers 45:427-434). Oligos with self-complementary sequences tend to form aggregates, and this formation can complicate HPSF and HPLC purification.
What is synthesis scale and synthesis yield?
The synthesis scale indicates the amount of starting material
(CPG) of the solid phase synthesis process.
Not to be confused with the synthesis yield, which is depending
on the coupling efficiency during synthesis.
The coupling efficiency again depends on raw material quality.
The individual sequence and length of an oligonucleotide also
influences the coupling efficiency and therefore the synthesis
yield as shown in the table below:
| |
Yield
CE 99.5% |
Yield
CE 99.0% |
Yield
C E 98.5% |
| 20mer |
91 |
83 |
75 |
| 30mer |
86 |
75 |
65 |
| 40mer |
82 |
68 |
55 |
| 60mer |
74 |
55 |
41 |
| 100mer |
61 |
37 |
22 |
Theoretical yield of full length product = coupling efficiency (CE)
(length of oligonucleotide-1)
What does OD mean?
One OD260 (optical density) unit of DNA is the amount
of DNA that gives an absorbance reading of 1.0 at a wavelength of
260 nm for a sample dissolved in 1.0 ml total volume of
ddH2O, read in a 1 cm quartz cuvette. The OD value is
used to determine the concentration of an unknown oligo solution by
measuring the absorption at 260 nm.
1 OD260 corresponds to approx. 33 µg/ml of single
stranded DNA.
Why is the oligo yield not reproducible every time?
Many factors can influence the yield. It depends on sequence
composition and oligo length.
The main reason why you might see different yields for the same
oligo (same sequence, same length), might be due to the column
loading with CPG (controlled pore glass) material, used to initiate
the synthesis. The minimum loading corresponds to the synthesis
scale but fluctuation of the CPG amount and CPG loading can occur,
which can lead to varying yields.
How long are unmodified oligonucleotides stable and how should I store them?
Our stability study shows that unmodified oligos are stable > 6 years by storing them lyophilised at -20 °C or below:
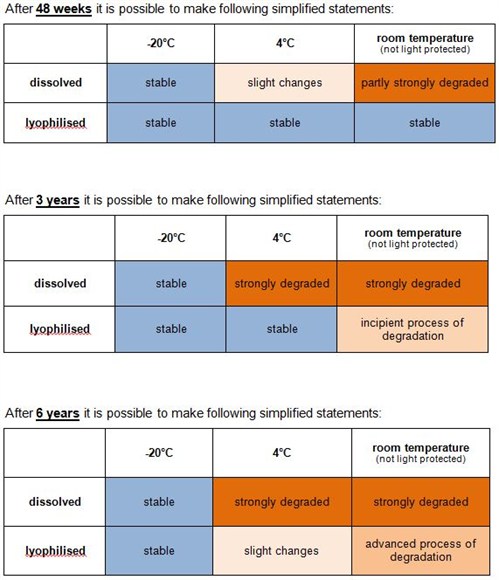
The stability study has been performed using the analytical methods HPLC and MALDI-TOF MS as well as application based in DNA sequencing.
Once you have dissolved your oligonucleotides in sterile water or buffered solutions (or you have already received your oligonucleotide in the requested solution), the best way to store them is to prepare aliquots of several tubes, to lyophilise the aliquots, and to store them at -20 °C.
The sample which is currently in use can be kept in a refrigerator at +4° C for a short time to avoid continuous freezing and thawing of the solution.
What is the shelf life of oligonucleotides?
The shelf life of an oligonucleotide is determined by three factors.
1. Sensitivity to pH
In contrast to double strand DNA, single strand oligonucleotides have a significantly higher sensitivity to acidic pH values. Therefore, it is recommended that the media used for resuspension of the oligonucleotides lies in the basic pH range.
2. Nuclease degradation
There is a danger of degradation of the oligonucleotides through nuclease activity. This nuclease degradation is hastened by the presence of salts, in particular by two-valent and three-valent ions. Avoiding the introduction of such nucleases by taking suitable hygiene measures at the workplace, and the addition of low amounts of ethylenediamine tetra-acetic acid (EDTA) can be helpful.
3. Freeze/thaw sensitivity
Single stranded oligos can be affected by freezing and thawing cycles. Keeping these cycles to a minimum will ensure oligo integrity. Dispensing the master stock of oligo upon receipt into usable aliquots is good practice to reduce repeated handling.
How can I optimise the shelf life of oligos?
Recommended resuspension buffer
For the stock solution we recommend 10 mM Tris-EDTA pH 8.0.
To make the working solution, we recommend to dilute the stock solution with Tris-buffer. This lowers the EDTA concentration that may disturb in enzymatical reactions.
The buffer concentration should be adjusted to the corresponding amount of oligonucleotides as DNA has a relatively high acid strength of its own. This buffer is equivalent to the buffer systems that are currently used for molecular biological applications such as PCR or sequencing.
We do not recommend dissolving our Salt Free and HPSF oligonucleotides unbuffered in distilled water. Due to the salt free properties of these oligos, no buffer effect is possible.
In addition, we recommend regularly checking the distilled water quality which is used for the production of the buffer.
Avoiding multiple thawing and freezing processes
After resuspension with the Tris-EDTA solution, aliquots of the oligonucleotides should be prepared and stored at -20°C. Individual aliquots can then be kept in liquid form at +4°C if they will be consumed within 1-2 days.
If long-term storage is required (> ½ year), it is recommended to store the samples at -80°C free of water (lyophilised) to exclude any enzymatic activities. Long-term storage at -20°C is not adequate because the exclusion of any enzymatic reaction can't be guaranteed.
From our experience, oligonucleotides can be stored between one month and two years depending on their individual properties and their different intended purposes and handling.
Because of the variations in oligo properties, applications, and handling, we can not give a definite shelf life guarantee.
How to choose the right dye?
One of the most common uncertainties when starting a new assay is how to select the best fluorescent label from all of the available options.
An optimal selection is based on reviewing the spectral properties of each dye, in relation to the instrument to be used. Each instrument has features to consider such as excitation source, optical filters, and sensitivity.
The most important dye characteristics to review when choosing an appropriate dye, are absorption- and emission spectra, the fluorescence Stokes shift, the extinction coefficient, pH-sensitivity, stability to photobleaching as well as the quantum yield.
Which criteria are to be considered for choosing the right dye?
Absorption- and emission spectra of fluorescent dyes
The absorbance of a dye quantifies how much of visible or UV light is absorbed by it. The emission value shows at which wavelength a fluorescent dye sends light respective radiation out. Each fluorescent dye has its own absorption- and emission value (see schedule below). The choice of the right absorption-/ emission spectra of a dye depends on the individual assay as well on the type of instrument that is used.
Stokes shift
The difference in energy between the excitation and emission maximum is known as the Stokes shift. With fluorescent dyes, a large Stokes shift is often desirable when optical filters are used to separate exciting light and fluorescence emission.
Extinction coefficient
This value is a direct measurement of the dye's ability to absorb light. The ability to absorb light will clearly have an effect on the amount of light it is able to emit.
pH sensitivity
Some fluorophores are more sensitive to alkaline or acid pH conditions. For example, in alkaline solution above pH 9 some dye molecules could degrade. Other fluorophores are stable in alkaline as well as in acid pH ranges. One of the reasons for this is the structural characteristic of the molecules.
Stability to photobleaching
Photobleaching is the photochemical destruction of a fluorophore, which may impact the observation of fluorescent molecules, since they will eventually be destroyed by a constant light exposure. The loss of dye intensity respective of the quantum yield during experiments can produce erroneous results. However, it is important to consider your application and the instrument as well. For instance, stability to photobleaching is not as important for DNA sequencers and flow cytrometry as it is for fluorescence microscopy.
Fluorescence quantum yield
The quantum yield of a radiation induced process is the number of times that a defined event occurs per photon absorbed by the system. The fluorescence quantum yield (also quantum efficiency) gives the efficiency of the fluorescence process. It is defined as the ratio of the number of photons emitted to the number of photons absorbed. Generally, the maximum fluorescence quantum yield is 1.0 (100%).
The quantum yield of fluorescent labels strongly depends on the current existing microenvironment such as pH-value, type of solution, concentration or temperature. Therefore specific quantum yields of single fluorescent dyes can not be revealed.
Can I have different modifications in one oligonucleotide?
We offer more than 100 different modifications and provide
oligos with multiple modifications or combinations of various
modifications. Please be aware that the yield of oligos with
multiple modifications is usually lower than those with fewer
modifications.
The more you increase the number of modifications, combinations of
modifications and the sequence length, the more complex the
synthesis and purification of your oligonucleotide becomes. If you
want to order multiple modified oligonucleotides, please contact our customer support team for
advice.
Can I have different backbone modifications in one
oligonucleotide?
Yes, all kinds of combinations of DNA and phosphorothioate (PTO)
bases are possible.
How should fluorescent oligonucleotides be stored?
For the stock solution we recommend a 10 mM Tris-HCl, pH 8; 1mM
EDTA dilution buffer for most modified oligos.
Oligos labelled with Cyano dyes (Cy3, CY3.5, Cy5 and Cy5.5) are
only stable at pH 7.0. A higher pH would damage the dye
molecule.
To make the working solution, we recommend to dilute the stock
solution with Tris-buffer. This lowers the EDTA concetration that
may disturb in enzymatical reactions.
In addition, we recommend regularly checking the distilled water
quality which is used for the production of the buffer.
The best way to store the oligos is to aliquot them into several
tubes, and store the aliquots at -20°C. The sample in current use
can be kept in a refrigerator at +4°C for a short time to avoid
continuous freezing and thawing of the solution.
Additionally, we strongly recommend protecting dye labelled
oligonucleotides from light.
How should I resuspend my oligonucleotides in tubes and in plates?
For the stock solution we recommend 10 mM Tris-EDTA pH 8.0.
We do not recommend dissolving our Salt Free and HPSF oligonucleotides unbuffered in distilled water. Due to the salt free properties of these oligos, no buffer effect is possible.
In addition, we recommend regularly checking the distilled water quality which is used for the production of the buffer.
To make the working solution, we recommend diluting the stock solution with Tris-buffer. This lowers the EDTA concentration that may disturb in enzymatic reactions.
The buffer concentration should be adjusted to the corresponding amount of oligonucleotides as DNA has a relatively high acid strength of its own.
The standard concentration for stock solutions is 100 µM. To obtain a concentration of 100 µM the synthesis report provides you with the appropriate diluents' volume.
For your convenience please use our Oligo Analysis Tool.
How do I anneal complementary oligos?
- Dissolve the lyophilised oligonucleotide in water or TE. Use
the concentrations of your oligonucleotide solutions stated on the
oligonucleotide synthesis report.
- Add the following components together:
Stock
Final Concentration
Oligonucleotide
1
100 nmole/ml
Oligonucleotide
2
100 nmole/ml
10x annealing
buffer*
1x
Nuclease-free
water
to appropriate volume
*10x annealing buffer: 100 mM Tris-HCI, pH 7.5,1 M NaCI and 10 mM
EDTA.
-
Heat the oligo solution to a temperature 10°C higher than the
calculated melting temperature. Maintain the temperature for 10
minutes. Remove the solution from the heating block/water bath and
allow it to cool slowly to room temperature on the bench
(approximately 1 hour).
-
Store the annealed oligos at -20°C as recommended.
How should Thiol-modified oligos be treated?
We recommend to reduce the thiol before using the oligo in your application:
1. Add 200 µl 10mM TCEP (solve 2,9mg TCEP-HCL in 1ml 1xTE buffer, pH 8.0) to your oligo
2. Shake it for 60 min at room temperature
3. Precipitate the oligo:
- Add 150 µl 3 M Na-Acetate [24,6 g Na-Acetate + 0,21 g Mg-Acetate in 100 ml dest. H2O]
- Fill tube with ethanol p.a.
- Shake gently
- Incubate 20 min at –20°C
4. Spin for 5 min at 13000 rpm, discard supernatant
5. Dry pellet at room temperature